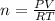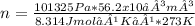Chemistry, 23.07.2019 17:00 elliedeegan3910
Asample of argon gas at stp occupies 56.2 liters. determine the number of moles of argon and the mass in the sample. for the above problem how will you rearrange the ideal gas law to solve for moles of argon?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1


Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1
You know the right answer?
Asample of argon gas at stp occupies 56.2 liters. determine the number of moles of argon and the mas...
Questions

Mathematics, 09.12.2020 04:30

Mathematics, 09.12.2020 04:30

Mathematics, 09.12.2020 04:30

Social Studies, 09.12.2020 04:30


Chemistry, 09.12.2020 04:30


English, 09.12.2020 04:30


Social Studies, 09.12.2020 04:30

Mathematics, 09.12.2020 04:30

Mathematics, 09.12.2020 04:30



Mathematics, 09.12.2020 04:30


Mathematics, 09.12.2020 04:30

Arts, 09.12.2020 04:30

Arts, 09.12.2020 04:30