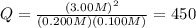Chemistry, 21.07.2019 23:00 falldownguyss
This system has an equilibrium constant of 50.5 at 448°c: h2(g) + i2(g) ↔ 2hi(g). what is the reaction quotient, q, for this system when [h2] = 0.200 m, [i2] = 0.100 m, and [hi] = 3.00 m?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1
You know the right answer?
This system has an equilibrium constant of 50.5 at 448°c: h2(g) + i2(g) ↔ 2hi(g). what is the react...
Questions














Computers and Technology, 16.03.2020 19:34


Mathematics, 16.03.2020 19:34


English, 16.03.2020 19:35


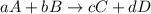
![Q=\frac{[C]^{c}[D]^{d}}{[A]^{a}[B]^{b}}](/tpl/images/0117/2709/a6d96.png)
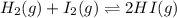
![Q=\frac{[HI]^{2}}{[H_{2}][I_{2}]}](/tpl/images/0117/2709/db0f2.png)