

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
A135 g sample of carbon disulfide requires 43.2 kj of heat to vaporize completely. what is the entha...
Questions

Physics, 03.06.2021 20:10




Physics, 03.06.2021 20:10



Geography, 03.06.2021 20:10

Mathematics, 03.06.2021 20:10


Mathematics, 03.06.2021 20:10




History, 03.06.2021 20:10





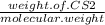
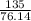
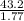 = 24.4 kj/mol
= 24.4 kj/mol

