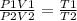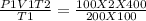Chemistry, 21.07.2019 11:00 tiakimberly3
Agas occupies 2.0 m3 at 100.0k and exerts a pressure of 100.0kpa. what volume will the gas occupy if the temperature is increased to 400.0 k and the pressure is increased to 200.0kpa

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Agas occupies 2.0 m3 at 100.0k and exerts a pressure of 100.0kpa. what volume will the gas occupy if...
Questions

Mathematics, 30.10.2020 07:20







Mathematics, 30.10.2020 07:30


Geography, 30.10.2020 07:30

History, 30.10.2020 07:30

Mathematics, 30.10.2020 07:30


Computers and Technology, 30.10.2020 07:30

Mathematics, 30.10.2020 07:30





English, 30.10.2020 07:30