
Engineering, 17.07.2021 03:00 sewolf1234
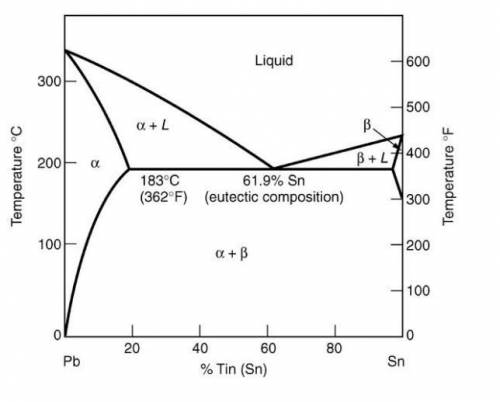
Use the equilibrium phase diagram on the following page to answer the following three questions. For each question, assume that the original solder composition is 30 wt% Sn.
1.What is the approximate average Sn content of the solid that has formed at 400°F?
2.What is the proportion of solid at 400°F?
3.What proportion of the final microstructure will be eutectic?

Answers: 3
Another question on Engineering

Engineering, 04.07.2019 18:10
Calculate the bore of a cylinder that has a stroke of 18 inches and an extension time of 6 seconds at a flow rate of 4 gal/min.
Answers: 3

Engineering, 04.07.2019 18:10
Atmospheric air has a temperature (dry bulb) of 80° f and a wet bulb temperature of 60° f when the barometric pressure is 14.696 psia. determine the specific humidity, grains/lb dry air. a. 11.4 c. 55.8 d. 22.5 b. 44.1
Answers: 1

Engineering, 04.07.2019 18:10
Ahot wire operates at a temperature of 200°c while the air temperature is 20°c. the hot wire element is a tungsten wire of 5 um diameter and 2 mm in length. plot using excel current, heat transfer and heat generated by the wire for air velocity varying from 1-10 m/s in steps of lm/s? matlab the sensor voltage output, resistance, or assume nu 0.989 re033pr13 take air properties at tr (200°c20°c)/2 = 110°c properties of tungsten: c 0.13 kj/kg.k 3 p 19250 kg/m k (thermal conductivity) = 174 w/m.k
Answers: 2

Engineering, 04.07.2019 18:10
Courses that are developed by subject matter experts, internal or extemal to the college or university. these programs are marketed by the school (clo2) marks a)-vocational schools b)-vendor training c)-colleges & universities d)-continuing education programs
Answers: 2
You know the right answer?
Use the equilibrium phase diagram on the following page to answer the following three questions. For...
Questions





History, 25.02.2020 22:27

Biology, 25.02.2020 22:27



Chemistry, 25.02.2020 22:27







Chemistry, 25.02.2020 22:27




Computers and Technology, 25.02.2020 22:28



