Mathematics, 27.02.2020 19:26 nickwwe13
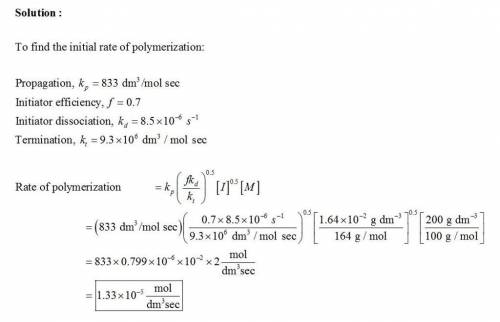
Methyl methacrylate was polymerized at a mass concentration of 200 g/dm3 in toluene using azobisisobutyronitrile (AIBN) as initiator at a mass concentration of 1.64 x 10-2 g/dm3 and a reaction temperature of 60°C. Calculate the initial rate of polymerization and the number average molar mass of poly(methyl methacrylate) formed in the initial stages of the reaction given that the relevant rate coefficients at 60°C are:
Initiator dissociation, kd = 8.5 x 10-6 s-1
Propagation, kp = 833 dm3 mol-1 s-1
Termination, kt = 9.3 x 106 dm3 mol-1 s-1
Transfer to monomer, ktrM = 3.93 x 10-3 dm3 mol-1 s-1
Transfer to solvent, ktrS = 7.34 x 10-3 dm3 mol-1 s-1
Initiator efficiency, f = 0.7 Termination by combination is negligible Density of initial solution of methyl methacrylate in toluene is 860 g/dm3

Answers: 1
Another question on Mathematics

Mathematics, 21.06.2019 17:00
Drag the tiles to the correct boxes to complete the pairs. match the cart-coordinates with their corresponding pairs of y-coordinates of the unit circle
Answers: 3


Mathematics, 21.06.2019 19:30
John checked his watch and said that it is thursday, 7 am. what will the day and time be 2006 hours plzzz i will give you 100 points
Answers: 1

Mathematics, 21.06.2019 21:00
Calculate the missing value. round the answer to on decimal place. start with 70, increase it by 21%, and end up with
Answers: 2
You know the right answer?
Methyl methacrylate was polymerized at a mass concentration of 200 g/dm3 in toluene using azobisisob...
Questions

Chemistry, 03.10.2021 14:00


Law, 03.10.2021 14:00




Mathematics, 03.10.2021 14:00


Mathematics, 03.10.2021 14:00




Chemistry, 03.10.2021 14:00

Mathematics, 03.10.2021 14:00



Chemistry, 03.10.2021 14:00



Mathematics, 03.10.2021 14:00