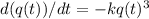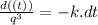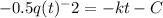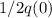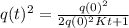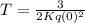Physics, 12.07.2019 02:30 thanks5640
Let q(t) represent the amount of a certain reactant present at time t. suppose that the rate of decrease of q(t) is proportional to q3(t). that is, q =−kq3, where k is a positive constant of proportionality. how long will it take for the reactant to be reduced to one half of its original amount? recall that, in problems of radioactive decay where the differential equation has the form q =−kq, the half-life was independent of the amount of material initially present. what happens in this case? does half-life depend on q(0), the amount initially present?

Answers: 1
Another question on Physics

Physics, 21.06.2019 16:20
Describe how the fermi level changes with donor level, temperature and contact with p and n type semiconductors
Answers: 1

Physics, 22.06.2019 02:30
Agas contained within a piston-cylinder assembly undergoes three processes in series: process 12: compression with pv= constant from 1 bar and 1 liter to 4 bar. process 23: constant pressure expansion to 1 liter. process 31: constant volume calculate the pressure and volume at each state, and sketch the processes on a p-vdiagram labeled with pressure and volume values at each numbered stat
Answers: 2

Physics, 22.06.2019 13:10
Most short-period comets do not have randomly oriented orbits because
Answers: 2

Physics, 22.06.2019 15:30
What is the increase in density of a medium due to wave travel?
Answers: 2
You know the right answer?
Let q(t) represent the amount of a certain reactant present at time t. suppose that the rate of decr...
Questions




Biology, 03.12.2021 17:30

English, 03.12.2021 17:30





Chemistry, 03.12.2021 17:30








Biology, 03.12.2021 17:30