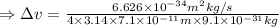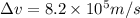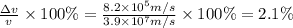Physics, 29.06.2019 04:00 avalonr2003
An atom of argon has a radius of 71.pm and the average orbital speed of the electrons in it is about ×3.9107/ms. calculate the least possible uncertainty in a measurement of the speed of an electron in an atom of argon. write your answer as a percentage of the average speed, and round it to 2 significant digits.

Answers: 1
Another question on Physics

Physics, 22.06.2019 14:30
Multiply or divide to make this english distance conversion. 174 inches = feet (14.5, 58, 2,088, 23)
Answers: 2

Physics, 22.06.2019 18:30
Asmall grinding wheel has a moment of inertia of 4.0×10−5 kg⋅m2 . what net torque must be applied to the wheel for its angular acceleration to be 150 rad/s2 ?
Answers: 3

Physics, 22.06.2019 19:30
Acamcorder has a power rating of 20 watts. if the output voltage from its battery is 9 volts, what current does it use?
Answers: 2

Physics, 23.06.2019 00:00
You are standing on a boat. which of the following strategies will make the boat start moving? a. pushing the mast b. throwing some cargo out of the boat c. pushing the front ofthe boat d. pushing another passenger
Answers: 3
You know the right answer?
An atom of argon has a radius of 71.pm and the average orbital speed of the electrons in it is about...
Questions

Mathematics, 03.02.2021 21:30

Geography, 03.02.2021 21:30




Advanced Placement (AP), 03.02.2021 21:30


Mathematics, 03.02.2021 21:30




Mathematics, 03.02.2021 21:30

Mathematics, 03.02.2021 21:30

Mathematics, 03.02.2021 21:30

Mathematics, 03.02.2021 21:30





Mathematics, 03.02.2021 21:30