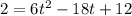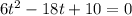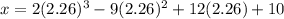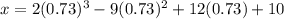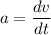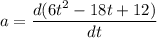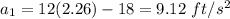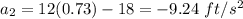Physics, 05.07.2019 21:30 joelwilliamjoel
The motion of a particle is defined by the relation x = 2t3 – 9t2 +12t +10, where x and t are expressed in feet and seconds, respectively. determine the time, position, and acceleration of the particle when v = 2.00 ft/s. (round the final answer to two decimal places.) the time, the position, and the acceleration of the particle when v = 2.00 are:

Answers: 1
Another question on Physics

Physics, 21.06.2019 13:30
An air bubble of volume 20 cm³ is at the bottom of a lake 40 m deep, where the temperature is 4.0°c. the bubble rises to the surface, which is at a temperature of 20°c.take the temperature of the bubble’s air to be the same as that of the surrounding water. just as the bubble reaches the surface, what is its volume?
Answers: 1

Physics, 21.06.2019 19:50
An object is dropped from a tower, 400 ft above the ground. the object's height above ground t seconds after the fall is s(t)equals400 minus 16 t squared. determine the velocity and acceleration of the object the moment it reaches the ground. the velocity of the object the moment it reaches the ground is nothing ft/s.
Answers: 1

Physics, 21.06.2019 22:40
Consider two metallic rods mounted on insulated supports. one is neutral, the other positively charged. you bring the two rods close to each, but without contact, and briefly ground the the neutral rod by touching it with your hand. show answer correct answer what would be resulting charge (if any) on the initially neutral rod
Answers: 1

Physics, 22.06.2019 07:10
1. how much energy is needed to raise the temperature of 40.0 g of argon from 25c to 40c? the specific heat capacity of argon is 0.520 j/(g·k) 2a. 23.0 ml of 0.100 m hcl (standard) are added from a buret to neutralize 50.0 ml of an unknown basic solution. 2b. if the oh- produced in the previous reaction came from ca(oh)2, then what is the molarity of the ca(oh)2? 3.calculate the new freezing-point of a solution when 60.5 grams of cacl2 solute is dissolved in 0.612 kg of water. 4. what is the maximum number of moles of alcl3 that can be produced from 5.0 mol al and 6.0 mol cl2? 5. a sample of oxygen gas has a volume of 150 ml when its pressure is 0.923 atm. if the pressure is increased to 0.987 atm and the temperature remains constant, what will the new volume be? 6. nitrogen gas in a closed container at a temperature of 100.0 oc and 3.0 atm is heated to 300 oc. what is the pressure of the gas at the higher temperature?
Answers: 3
You know the right answer?
The motion of a particle is defined by the relation x = 2t3 – 9t2 +12t +10, where x and t are expres...
Questions





Biology, 24.09.2019 21:30


Biology, 24.09.2019 21:30




Health, 24.09.2019 21:30



Mathematics, 24.09.2019 21:30

Mathematics, 24.09.2019 21:30


Biology, 24.09.2019 21:30



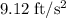 and 14.23 ft respectively.
and 14.23 ft respectively.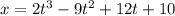 .
.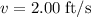 .
.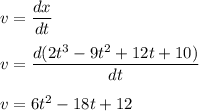
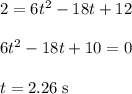
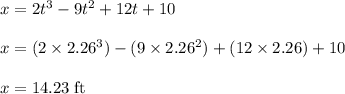
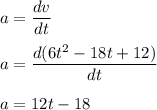
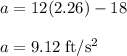
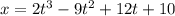 ........(1)
........(1)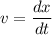
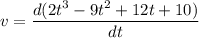
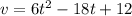 ............(2)
............(2)