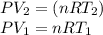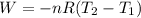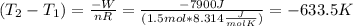Physics, 31.07.2019 03:10 ronalescobar2002
One and one-half moles of an ideal monatomic gas expand adiabatically, performing 7900 j of work in the process. what is the change in temperature of the gas during this expansion?

Answers: 3
Another question on Physics

Physics, 21.06.2019 23:30
Edward tolman explained the results of his study by theorizing that the rats were learning about the maze during every trial but they a. were agitated because other groups were getting reinforcement b. could not remember how to demonstrate it without reinforcement c. were not motivated to demonstrate it without reinforcement d. seemed to be too lazy to actually work without reinforcement
Answers: 3

Physics, 22.06.2019 21:30
Calculate the minimum energy required to remove one proton from the nucleus 126c. this is called the proton-removal energy. (hint: find the difference between the mass of a 126c nucleus and the mass of a proton plus the mass of the nucleus formed when a proton is removed from 126c.) express your answer with the appropriate units. emin e m i n = nothing nothing request answer part b how does the proton-removal energy for 126c compare to the binding energy per nucleon for 126c, calculated using eb=(zmh+nmn−azm)c2?
Answers: 1

Physics, 23.06.2019 03:30
Use the ratio form of kepler’s third law, = , and the data provided to determine the time it takes mars to orbit the sun. round your answer to the nearest tenth. earth’s orbital period = 1.0 earth year earth’s distance from the sun = 1.0 au mars’s distance from the sun = 1.5 au it takes mars about ⇒ 1.8 earth years to orbit the sun.
Answers: 2

Physics, 23.06.2019 04:50
By examining a wave’s pattern after it interacts with a barrier or gap, measurements can be made to better understand certain wave phenomena. what is a broad question you can answer by doing this experiment?
Answers: 2
You know the right answer?
One and one-half moles of an ideal monatomic gas expand adiabatically, performing 7900 j of work in...
Questions


Mathematics, 16.07.2019 23:00

Mathematics, 16.07.2019 23:00

Chemistry, 16.07.2019 23:00


Advanced Placement (AP), 16.07.2019 23:00



History, 16.07.2019 23:00



Chemistry, 16.07.2019 23:00

Chemistry, 16.07.2019 23:00

Mathematics, 16.07.2019 23:00




Computers and Technology, 16.07.2019 23:00

Advanced Placement (AP), 16.07.2019 23:00

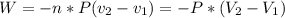 Where 2 and 1 subscripts mean the final and the initial state respectively. The equation negative sign says that for an expansion of the gas, the system is making work, so the energy is going out of the system.
Where 2 and 1 subscripts mean the final and the initial state respectively. The equation negative sign says that for an expansion of the gas, the system is making work, so the energy is going out of the system.