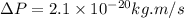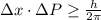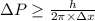Rutherford's scattering experiments gave the first indications that an atom consists of a small, dense, positively charged nucleus surrounded by negatively charged electrons. his experiments also allowed for a rough determination of the size of the nucleus. in this problem, you will use the uncertainty principle to get a rough idea of the kinetic energy of a particle inside the nucleus. consider a nucleus with a diameter of roughly 5.0×10^−15 meters. consider a particle inside the nucleus. the uncertainty δx in its position is equal to the diameter of the nucleus. what is the uncertainty δp of its momentum? to find this, use δx δp ≥ h

Answers: 3
Another question on Physics

Physics, 21.06.2019 22:10
Agas is contained in a vertical, frictionless piston–cylinder device. the piston has a mass of 3.2 kg and a cross-sectional area of 35 cm2. a compressed spring above the piston exerts a force of 190 n on the piston. if the atmospheric pressure is 95 kpa, determine the pressure inside the cylinder.
Answers: 3

Physics, 22.06.2019 08:30
Hey student studies gravity using objects that have the same mass which two objects have the greatest gravitational force acting between them a. 100kg 1.0m 100kg b. 100kg 2.0m 100kg c. 100kg 2.0m 100kg big d. 100kg big 3.0m 100kg big
Answers: 1

Physics, 22.06.2019 09:00
A100 kg running back runs at 5m/s into a stationary linebacker. it takes 0.5 for the running back to be completely stopped
Answers: 3

You know the right answer?
Rutherford's scattering experiments gave the first indications that an atom consists of a small, den...
Questions






Chemistry, 31.08.2020 01:01


Computers and Technology, 31.08.2020 01:01

Mathematics, 31.08.2020 01:01

Computers and Technology, 31.08.2020 01:01



Mathematics, 31.08.2020 01:01


Biology, 31.08.2020 01:01



Mathematics, 31.08.2020 01:01


Chemistry, 31.08.2020 01:01