
Physics, 04.09.2019 23:10 biaxialpower789
In the bohr theory of the hydrogen atom, an electron moves in a circular orbit about a proton, where the radius of the orbit is approximately 0.522 ✕ 10-10 m. (the actual value is 0.529 ✕ 10-10 m.) (a) find the magnitude of the electric force exerted on each particle.
(b) if this force causes the centripetal acceleration of the electron, what is the speed of the electron?

Answers: 1
Another question on Physics

Physics, 22.06.2019 03:30
What makes thermal imaging cameras useful? they can detect differences in color. they can detect differences in wave speeds. they can detect differences in temperature. they can detect mechanical waves.
Answers: 1

Physics, 22.06.2019 05:30
The a992 steel rod bc has a diameter of 50 mm and is used as a strut to support the beam. determine the maximum intensity w of the uniform distributed load that can be applied to the beam without risk of causing the strut to buckle. take f.s. = 2 against bucklin
Answers: 3

Physics, 22.06.2019 11:30
(2) (a) you have a simple circuit that consists of only a battery (δvbat=1.5v) and two resistors with resistance r1=10ω and r2=5ω, connected in series with each other. what is the current running through the battery? (b) you re-arrange your circuit so now r2 is attached in parallel to r1 rather than in series. what is the current running through the battery? (c) you add an additional resistor r3=7ω on the same branch as r2. what is the current running through the battery? (d) what is the power dissipated in r3?
Answers: 3

Physics, 22.06.2019 19:30
If the area of a square has increased by a factor of 16, by how much has each side increased?
Answers: 3
You know the right answer?
In the bohr theory of the hydrogen atom, an electron moves in a circular orbit about a proton, where...
Questions



Mathematics, 03.07.2019 02:00


English, 03.07.2019 02:00


Mathematics, 03.07.2019 02:00

Mathematics, 03.07.2019 02:00









Mathematics, 03.07.2019 02:00

Mathematics, 03.07.2019 02:00

History, 03.07.2019 02:00

Biology, 03.07.2019 02:00

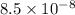 N
N  m/s
m/s = radius of the orbit = 0.522 x 10⁻¹⁰ m
= radius of the orbit = 0.522 x 10⁻¹⁰ m 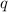 = magnitude of charge on each proton and electron = 1.6 x 10⁻¹⁹ C
= magnitude of charge on each proton and electron = 1.6 x 10⁻¹⁹ C 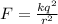
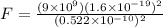
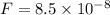 N
N 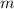 = mass of electron = 9.1 x 10⁻³¹ kg
= mass of electron = 9.1 x 10⁻³¹ kg  = speed of electron = ?
= speed of electron = ?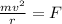
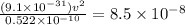
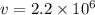 m/s
m/s

