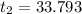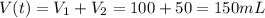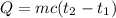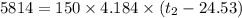Physics, 08.10.2019 04:00 Kingdcn6261
A100.0 ml sample of 1.020 m hcl is mixed with a 50.0 ml sample of 2.040 m naoh in a styrofoam cup. if both solutions were initially at 24.53°c, and the enthalpy of the neutralization reaction is −57 kj/mole of h2o formed, what is the final temperature of the mixture? assume that the solution has a density of 1.00 g/ml and a specific heat of 4.184 j/g°c, and that the styrofoam cup has an insignificant heat capacity.

Answers: 2
Another question on Physics

Physics, 22.06.2019 10:30
Light from a sodium lamp passes through a diffraction grating that has 1000 slits per millimeter. the interference pattern is viewed on a screen 1.000 m behind the grating. the first (m = 1) two bright yellow fringes that are visible are 0.7288 m and 0.7300 m from the central maximum. what are the wavelengths of these two fringes?
Answers: 2


Physics, 22.06.2019 14:00
The earth exerts a gravitational force of 500 n on amy. what is amy’s mass in kg?
Answers: 2

Physics, 22.06.2019 15:00
Mechanical energy is when the amount of kinetic and potential energies added together remains the same. conserved created lost
Answers: 1
You know the right answer?
A100.0 ml sample of 1.020 m hcl is mixed with a 50.0 ml sample of 2.040 m naoh in a styrofoam cup. i...
Questions





Computers and Technology, 26.11.2019 06:31


Computers and Technology, 26.11.2019 06:31

Chemistry, 26.11.2019 06:31


Social Studies, 26.11.2019 06:31









Computers and Technology, 26.11.2019 06:31