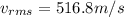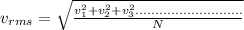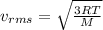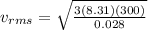Because the kinetic energy of a single molecule is related to its velocity squared, the best measure of the kinetic energy of the entire gas is obtained by computing the mean squared velocity, (v2)avg, or its square root vrms. the quantity vrms is more common than (v2)avg because it has the dimensions of velocity instead of the less-familiar velocity-squared. what is the rms speed vrms of the molecules in the nitrogen gas?

Answers: 2
Another question on Physics


Physics, 23.06.2019 01:30
Match each type of lever with the correct diagram. 1. first-class 2. third-class 3. second-class
Answers: 3

Physics, 23.06.2019 09:00
Consider motion in one dimension. (the sign of the vector quantities is their direction indicator.) an object moves in the positive x-direction with speed 2.9 m/s for 4.5 s. it stops for 3.8 s and then moves in the negative x direction with speed 2.8 m/s for 4 s. what is the total distance traveled by the object in units of meter? enter a number with two digits behind the decimal point.
Answers: 1

You know the right answer?
Because the kinetic energy of a single molecule is related to its velocity squared, the best measure...
Questions


Social Studies, 07.03.2020 04:56

Chemistry, 07.03.2020 04:57















Computers and Technology, 07.03.2020 04:57