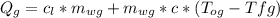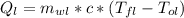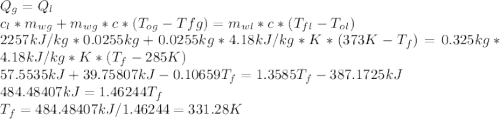Physics, 24.10.2019 00:50 perezsamantha3oqr0za
Amass of 25.5 g of h2o(g) at 373 k is mixed with 325 g of h2o(l) at 285 k and 1 atm. calculate the final temperature of the system once equilibrium has been reached. assume that ,m for h2o is constant at its values for 298 k throughout the temperature range of interest.

Answers: 3
Another question on Physics

Physics, 22.06.2019 09:30
Suppose you increase your walking speed from 7 m/s to 13 m/s in a period of 2 s. what is your acceleration?
Answers: 2

Physics, 22.06.2019 17:00
Amajor difference radio waves, visible light, and gamma rays is the of the photons, which results in different photon frequencies and wavelengths
Answers: 1

Physics, 22.06.2019 23:30
You have two photos of a person walking. one shows the person at the corner of third and main streets, the other shows the person at the corner of tenth and main streets. there are lampposts at every corner in this town, and the first picture shows it to be 10: 32: 00 exactly. the second picture shows it to be 10: 49: 30. you know three facts: (1) all of the clocks are synchronized; (2) there are exactly 12 equal-sized blocks per kilometer in this town; and (3) the streets that cross main in this area are numbered consecutively, with no interruptions. what is the person’s average speed in kilometers per hour?
Answers: 2

You know the right answer?
Amass of 25.5 g of h2o(g) at 373 k is mixed with 325 g of h2o(l) at 285 k and 1 atm. calculate the f...
Questions


Biology, 03.07.2019 09:30

Social Studies, 03.07.2019 09:30





Biology, 03.07.2019 09:30









Mathematics, 03.07.2019 09:30


English, 03.07.2019 09:30

English, 03.07.2019 09:30