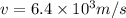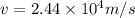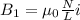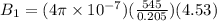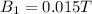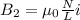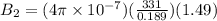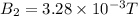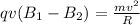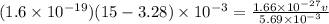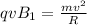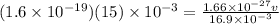Physics, 19.11.2019 19:31 savannahsharp5981
Two solenoids are nested coaxially such that their magnetic fields point in opposite directions. treat the solenoids as ideal. the outer one has a radius of 20 mm, and the radius of the inner solenoid is 10 mm. the length, number of turns, and current of the outer solenoid are, respectively, 20.5 cm, 545 turns, and 4.53 a. for the inner solenoid the corresponding quantities are 18.9 cm, 331 turns, and 1.49 a. at what speed, v1,should a proton be travelling, inside the apparatus and perpendicular to the magnetic field, if it is to orbit the axis of the solenoids at a radius of 5.69 mm? v1 =/s and at what speed, v2, for an orbital radius of 16.9 mm? v2 =/s

Answers: 1
Another question on Physics

Physics, 22.06.2019 12:50
Assume you measured the mass of the cart to be (500 ± 1) g and the mass of the additional mass you put on the cart to be (500 ± 1) g as well. since the scale you are using in the lab cannot measure objects heavier than 600g you will have to sum up individual pieces and propagate the error. so what would be the mass and the standard error of the cart and the mass
Answers: 3

Physics, 23.06.2019 01:30
The first law of thermodynamics is as follows: ∆u=q-w , which means in a system is equal to added, minus done by the system. the units for these variables are in .
Answers: 1

Physics, 23.06.2019 03:00
The stopwatch used by a student to measure velocity of a pulse in a slinky was of least count 0.1 second. he stops the stopwatch when a pulse has made 3 journeys from one end to the other of the slinky. he finds the seconds hand to be at 52nd division. calculate the correct time noted by him.
Answers: 3

Physics, 23.06.2019 09:30
How many milliliters of water at 23 °c with a density of 1.00 g/ml must be mixed with 180 ml (about 6 oz) of coffee at 95 °c so that the resulting combination will have a temperature of 60 °c? assume that coffee and water have the same density and the same specific heat. how much will the temperature of a cup (180 g) of coffee at 95 °c be reduced when a 45 g silver spoon (specific heat 0.24 j/g °c) at 25 °c is placed in the coffee and the two are allowed to reach the same temperature? assume that the coffee has the same density and specific heat as water. a 45-g aluminum spoon (specific heat 0.88 j/g °c) at 24 °c is placed in 180 ml (180 g) of coffee at 85 °c and the temperature of the two become equal. (a) what is the final temperature when the two become equal? assume that coffee has the same specific heat as water. (b) the first time a student solved this problem she got an answer of 88 °c. explain why this is clearly an incorrect answer.
Answers: 1
You know the right answer?
Two solenoids are nested coaxially such that their magnetic fields point in opposite directions. tre...
Questions




History, 04.09.2020 20:01

English, 04.09.2020 20:01

Mathematics, 04.09.2020 20:01

Mathematics, 04.09.2020 20:01

History, 04.09.2020 20:01







History, 04.09.2020 20:01


Mathematics, 04.09.2020 20:01