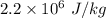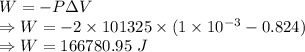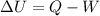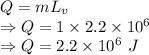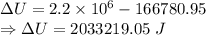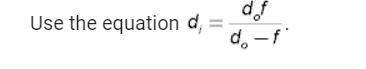Physics, 29.11.2019 04:31 lizdeleon248
When water is boiled under a pressure of 2.00atm, the heat of vaporization is 2.20×106j/kg and the boiling point is 120∘c. at this pressure, 1.00kg of water has a volume of 1.00×10−3m3, and 1.00 kg of steam has a volume of 0.824m3.
a)compute the work done when 1.00kg of steam is formed at this temperature
b)compute the increase in internal energy of the water.

Answers: 3
Another question on Physics

Physics, 22.06.2019 08:00
Which graph represents motion with an object with positive velocity that is located at a position of 3 meters at a time of 0 seconds? a) a b) bb eliminate c) c d) d
Answers: 1

Physics, 22.06.2019 09:30
1. how to locate the image in converging(concave) mirror and diverging (convex) mirror with salt. 2. how to locate the image in a converging (convex) lens and diverging (concave) lens with salt.
Answers: 1

Physics, 22.06.2019 10:30
J. j. thomson’s experiment disproved the theory that an atom
Answers: 3

Physics, 22.06.2019 11:00
What is the rate of 12 liters of water moving through a water hose in 4.0 minutes?
Answers: 1
You know the right answer?
When water is boiled under a pressure of 2.00atm, the heat of vaporization is 2.20×106j/kg and the b...
Questions











Computers and Technology, 03.04.2020 16:39









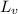 = Heat of vaporization =
= Heat of vaporization =