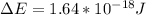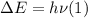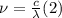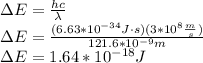Physics, 10.12.2019 06:31 alyssamaize
When an electron falls from a higher to a lower energy level in an atom, the photon released has a wavelength of 121.6 nm. what is the energy difference between the two energy levels, in j?

Answers: 3
Another question on Physics

Physics, 21.06.2019 23:10
6–55 refrigerant-134a enters the condenser of a residential heat pump at 800 kpa and 358c at a rate of 0.018 kg/s and leaves at 800 kpa as a saturated liquid. if the compressor consumes 1.2 kw of power, determine (a) the cop of the heat pump and (b) the rate of heat absorption from the outside air.
Answers: 2

Physics, 22.06.2019 10:30
Air is to be preheated by hot exhaust gases in a cross-flow heat exchanger before it enters the furnace. air enters the heat exchanger at 95 kpa and 20°c at a rate of 0.6 m^3/s. the combustion gases (cp = 1.10 kj/kg°c) enter at 160°c at a rate of 0.95 kg/s and leave at 95°c. determine the rate of heat transfer to the air and its outlet temperature.
Answers: 2


Physics, 23.06.2019 00:30
What is the speed vfinal of the electron when it is 10.0 cm from charge 1?
Answers: 1
You know the right answer?
When an electron falls from a higher to a lower energy level in an atom, the photon released has a w...
Questions







Mathematics, 07.11.2020 20:30



History, 07.11.2020 20:30



Spanish, 07.11.2020 20:30

Mathematics, 07.11.2020 20:30

Arts, 07.11.2020 20:30


English, 07.11.2020 20:30



Social Studies, 07.11.2020 20:40