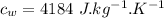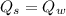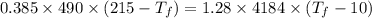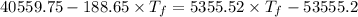Physics, 23.01.2020 00:31 simplydimps22owbohb
Heat transfer, specific heat. and calorimetrya 1.28-kg sample of water at 10.0 "c is in a calorimeter. you drop a piece of steel with a mass of 0.385 kg at 215 "c into it. after the sizzling subsides, what is the final equilibrium temperature? (make the reasonable assumptions that any steamproduced condenses into liquid water during the process of equilibration and that the evaporation and condensation don’taffect the outcome. as we’ll see in the next section.)

Answers: 1
Another question on Physics

Physics, 21.06.2019 16:00
Lets say that an object of a certain volume has a certin mass. what happens to its mass when you double its volume?
Answers: 3

Physics, 21.06.2019 22:40
The desk has a weight of 75 lb and a center of gravity at g. determine the initial acceleration of a desk when the man applies enough force f to overcome the static friction at a and b. also, find the vertical reactions on each of the two legs at a and at b. the coefficients of static and kinetic friction at a and b are ms = 0.5 and mk = 0.2, respectively
Answers: 2

Physics, 22.06.2019 04:50
Describe the function of the endomembrane system? what organelles are involved?
Answers: 3

Physics, 22.06.2019 06:00
Which of the following changes will result in a stronger electromagnet? a. using fewer coils of wire b. using a higher voltage c. using a shorter nail d. using a longer wire
Answers: 1
You know the right answer?
Heat transfer, specific heat. and calorimetrya 1.28-kg sample of water at 10.0 "c is in a calorimete...
Questions


Mathematics, 02.03.2021 21:30

Mathematics, 02.03.2021 21:30


Physics, 02.03.2021 21:30

Mathematics, 02.03.2021 21:30

Mathematics, 02.03.2021 21:30

Mathematics, 02.03.2021 21:30

Mathematics, 02.03.2021 21:30

English, 02.03.2021 21:30




Mathematics, 02.03.2021 21:30

Mathematics, 02.03.2021 21:30

Mathematics, 02.03.2021 21:30




Advanced Placement (AP), 02.03.2021 21:30


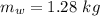 initial temperature of water,
initial temperature of water,  mass of steel,
mass of steel, 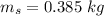 initial temperature of steel,
initial temperature of steel, 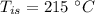 specific heat capacity of steel,
specific heat capacity of steel,  specific heat capacity of water,
specific heat capacity of water,