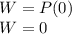A certain amount of ideal monatomic gas is maintained at constant volume as it is cooled from 455K to 405 K. This feat is accomplished by removing 400 J of heat from the gas. How much work is done by the gas during this process? The ideal gas constant is R = 8.314 J/mol • K.

Answers: 3
Another question on Physics

Physics, 21.06.2019 16:30
Acar accelerates from 5.0 m/s to to 11 m/s at a rate of 3.0 m/s^2. how far does it travel while accelerating? 20m 117m 43m 16m
Answers: 3

Physics, 22.06.2019 11:10
Consider an insulating crystal, made up of layers of atoms. what form would you expect the temperature dependence of the phonon heat capacity to approach at extremely low temperatures if the interlayer coupling is i)very strong (rigid coupling), and ii) very weak. explain.
Answers: 3

Physics, 22.06.2019 12:30
Which levels of government establish and implement educational requirements for minors
Answers: 1

Physics, 22.06.2019 20:50
An ideal otto cycle has a compression ratio of 8. at the beginning of the compression process, air is at 95 kpa and 27°c, and 750 kj/kg of heat is transferred to air during the constant-volume heat-addition process. assuming constant specific heats at room temperature, determine (a) the pressure and temperature at the end of the heat-addition process, (b) the net work output, (c) the thermal efficiency, and (d) the mean effective pressure for the cycle. (4390 kpa, 1730 k; 423 kj/kg; 56.4%; 534 kpa)
Answers: 1
You know the right answer?
A certain amount of ideal monatomic gas is maintained at constant volume as it is cooled from 455K t...
Questions


English, 09.05.2021 19:30

French, 09.05.2021 19:30

Social Studies, 09.05.2021 19:30

Mathematics, 09.05.2021 19:30

Social Studies, 09.05.2021 19:30

Chemistry, 09.05.2021 19:30

Physics, 09.05.2021 19:30

Mathematics, 09.05.2021 19:30

Physics, 09.05.2021 19:30

Mathematics, 09.05.2021 19:30

German, 09.05.2021 19:30



Business, 09.05.2021 19:30

Computers and Technology, 09.05.2021 19:30



Computers and Technology, 09.05.2021 19:30

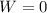
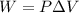
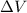 is the volume change. Since the gas is maintained at constant volume, we have
is the volume change. Since the gas is maintained at constant volume, we have 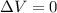 . So:
. So: