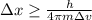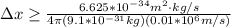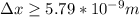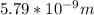German physicist Werner Heisenberg related the uncertainty of an object's position ( Δ x ) to the uncertainty in its velocity ( Δ v ) Δ x ≥ h 4 π m Δ v where h is Planck's constant and m is the mass of the object. The mass of an electron is 9.11 × 10 − 31 kg. What is the uncertainty in the position of an electron moving at 2.00 × 10 6 m/s with an uncertainty of Δ v = 0.01 × 10 6 m/s ?

Answers: 1
Another question on Physics


Physics, 22.06.2019 05:30
If gases like carbon dioxide and methane make up less than 1% of the total atmosphere, why is it important for scientists to monitor changes in percentages of these gases?
Answers: 1

Physics, 22.06.2019 16:00
Agroup of monkeys is trying to cross the river in a rowboat. the maximum buoyant force on the rowboat is 2,000 n. the weight of the rowboat is 1,000 n. each monkey has a weight of 150 n. how many monkeys can safely cross in the rowboat at one time? explain your reasoning, including any calculations you used to find the answer.
Answers: 1

Physics, 22.06.2019 22:10
M1 = 2.8 kg, m2 = 6.72 kg, m3 = 11.2 kg, byas in .is ,is .toof m3 it 0.91 m. (in m/s)
Answers: 3
You know the right answer?
German physicist Werner Heisenberg related the uncertainty of an object's position ( Δ x ) to the un...
Questions


World Languages, 26.02.2021 03:50


Social Studies, 26.02.2021 03:50



Computers and Technology, 26.02.2021 03:50






Health, 26.02.2021 03:50

English, 26.02.2021 03:50

Mathematics, 26.02.2021 03:50

English, 26.02.2021 03:50




Mathematics, 26.02.2021 03:50

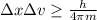
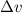 = Uncertainty in velocity of object
= Uncertainty in velocity of object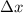 = Uncertainty in position of object
= Uncertainty in position of object