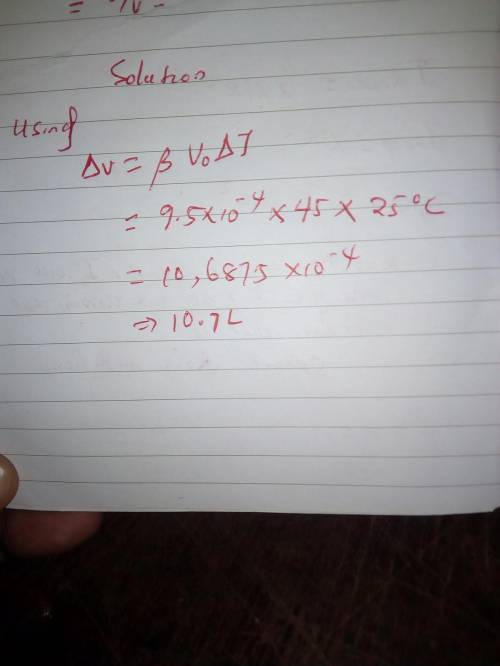An automobile fuel tank is filled to the brim with 45 L of gasoline (12 gal) at 10°C. Immediately afterward, the vehicle is parked in the sunlight, where the temperature is 35°C. How much gasoline overflows from the tank as a result of the expansion? (Neglect the expansion of the tank.)

Answers: 1
Another question on Physics

Physics, 21.06.2019 18:20
The extremely high temperatures needed to trigger nuclear fusion are proposed to be generated by laser irradiating a spherical pellet of deuterium and tritium fuel of diameter 1.8 mm. (a) determine the maximum fuel temperature that can be achieved by irradiating the pellet with 200 lasers, each producing a power of 550 w. the pellet has an absorptivity 0.3 and emissivity 0.8.
Answers: 2

Physics, 21.06.2019 20:10
Current 1 of 8.4 a runs for 240 seconds and then stops. current 2 is 10.5 a. how long does current 2 have to un to deliver the same amount of charge as current 1? 88.28 192 s 2016 s 21,000 s
Answers: 2

Physics, 22.06.2019 10:30
In science and physics what is the standard unit of measure for speed?
Answers: 1

Physics, 22.06.2019 14:20
4r-134a enters the condenser of a residential heat pump at 800 kpa and 50°c at a rate of 0.022 kg/s and leaves at 750 kpa subcooled by 3°c. the refrigerant enters the compressor at 200 kpa superheated by 4°c determine (a) the isentropic efficiency of the compressor, (b) the rate of heat supplied to the heated room, and (c) the cop of the heat pump. also, determine (d) the cop and rate of heat supplied to the heated room if this heat pump operated on the ideal vapor-compression cycle between the pressure limits of 200 and 800 kpa. (0.757, 4.37 kw, 5.12, 6.18, 3.91 kw)
Answers: 3
You know the right answer?
An automobile fuel tank is filled to the brim with 45 L of gasoline (12 gal) at 10°C. Immediately af...
Questions






Health, 24.01.2021 20:40


Mathematics, 24.01.2021 20:40

History, 24.01.2021 20:40

Mathematics, 24.01.2021 20:40


Mathematics, 24.01.2021 20:40

Mathematics, 24.01.2021 20:40


History, 24.01.2021 20:40

Mathematics, 24.01.2021 20:40


Chemistry, 24.01.2021 20:40