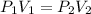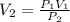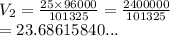Physics, 11.11.2020 20:10 qwertytown99
A student collects 25 mL of gas at 96 kPa. What volume would this gas occupy at 101.325 kPa. There is no change in temperature or mass.

Answers: 2
Another question on Physics

Physics, 22.06.2019 01:00
Calculate the amount of heat produced when 18000 coulombs of charge is transferred in one hour through a potential difference of 50v
Answers: 3

Physics, 22.06.2019 07:20
Use the information presented in the graph to answer the questions. which segments show acceleration? which segment indicates that the object is slowing down? what is the velocity of segment b? what is the acceleration of segment b?
Answers: 3

Physics, 22.06.2019 16:20
Amagnetic field applies forces on: a)static chargesb)moving chargesc)water flow
Answers: 1

Physics, 22.06.2019 17:00
In the future, people will only enjoy one sport: electrodisc. in this sport, you gain points when you cause metallic discs hovering on a field to exchange charge. you are an electrodisc player playing the popular four disc variant. the disks have charges of qa = −8.0 µc, qb = −2.0 µc, qc = +5.0 µc, and qd = +12.0 µc. (1) you bring two disks together and then separate them. you measure the resulting charge of these two disks and find that it is +5.0 µc per disk. which two disks did you bring together? (a) a and b (b) a and c (c)a and d (d)b and c(e) b and d (f) c and d. (2) you bring three disks together and then separate them. you measure the resulting charge of these three disks and find that it is +3.0 µc per disk. which three disks did you bring together? a, b, and c (a) a, b, and d (c) a, c, and d (d) b, c, and d. (3) given the resulting charge of each disk measured in (b) is +3.0 µc, how many electrons would you need to add to a disk of this charge to electrically neutralize it? electrons
Answers: 3
You know the right answer?
A student collects 25 mL of gas at 96 kPa. What volume would this gas occupy at 101.325 kPa. There i...
Questions

Mathematics, 03.02.2020 02:46


History, 03.02.2020 02:46

Biology, 03.02.2020 02:46

Health, 03.02.2020 02:46



German, 03.02.2020 02:46


Mathematics, 03.02.2020 02:46

History, 03.02.2020 02:46

Mathematics, 03.02.2020 02:46

Physics, 03.02.2020 02:46


Chemistry, 03.02.2020 02:46

History, 03.02.2020 02:46



Computers and Technology, 03.02.2020 02:46

Biology, 03.02.2020 02:46