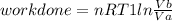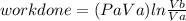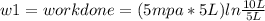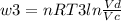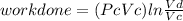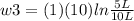Physics, 19.02.2021 17:00 bkimswift7
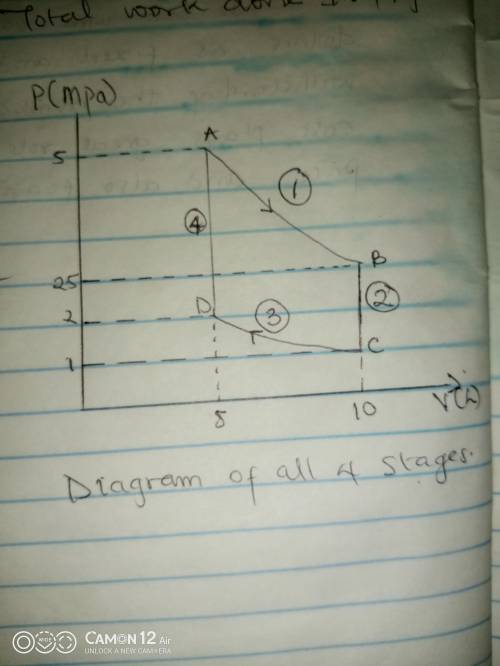
Thermodynamic Processes Two moles of a monatomic ideal gas at (5 MPa, 5 L) is expanded isothermally until the volume is doubled (step 1). Then it is cooled isochorically until the pressure is 1 MPa (step 2). The temperature drops in this process. The gas is now compressed isothermally until its volume is back to 5 L, but its pressure is now 2 MPa (step 3). Finally, the gas is heated isochorically to return to the initial state (step 4). (a) Draw the four processes in the pV plane. (b) Find the total work done by the gas.

Answers: 1
Another question on Physics

Physics, 21.06.2019 21:40
Since the investigative question has two variables, you need to focus on each one separately. thinking only about the first part of the question, mass, what might be a hypothesis that would illustrate the relationship between mass and kinetic energy? use the format of "if…then…because…” when writing your hypothesis.
Answers: 1

Physics, 22.06.2019 05:00
Modern physics a photon emitted from an excited hydrogen atom has an energy of 3.02 electron volts. which electron energy-level transition would produce this photon? a. n=1 to n=6 b. n=2 to n=6 c. n=6 to n=1 d. n=6 to n=2 i chose b but the correct answer is d can someone tell me why? and what's the difference?
Answers: 1

Physics, 22.06.2019 12:20
What is the coefficient of kinetic friction μk between the block and the tabletop?
Answers: 1

Physics, 22.06.2019 17:00
Amajor difference radio waves, visible light, and gamma rays is the of the photons, which results in different photon frequencies and wavelengths
Answers: 1
You know the right answer?
Thermodynamic Processes
Two moles of a monatomic ideal gas at (5 MPa, 5 L) is expanded isothermally...
Questions

Mathematics, 14.12.2020 04:10



English, 14.12.2020 04:10

Social Studies, 14.12.2020 04:10

Mathematics, 14.12.2020 04:10




Mathematics, 14.12.2020 04:10

Mathematics, 14.12.2020 04:10

Biology, 14.12.2020 04:10

Chemistry, 14.12.2020 04:10


Mathematics, 14.12.2020 04:10




Mathematics, 14.12.2020 04:10