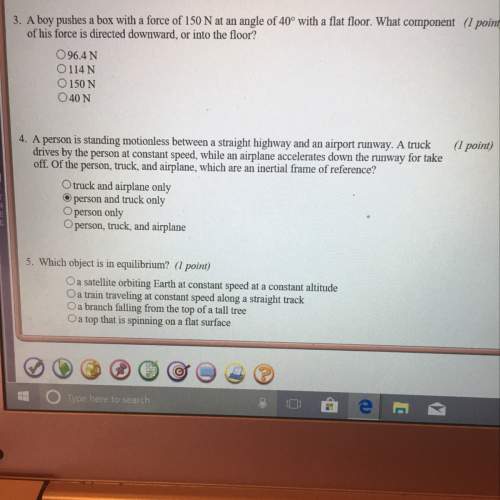
Describe how the amount of heat released or absorbed related to specific heat capacity and amount of it? a) amount of heat absorbed or released is doubled if quantity is doubled. if a different substance with half the specific heat capacity is used, the amount of heat absorbed or released is doubled. b) amount of heat absorbed or released is doubled if quantity is cut in half. if a different substance with half the specific heat capacity is used, the amount of heat absorbed or released is doubled. c) amount of heat absorbed or released is doubled if quantity is doubled. if a different substance with half the specific heat capacity is used, the amount of heat absorbed or released is cut in half. d) amount of heat absorbed or released is doubled if quantity is cut in half. if a different substance with half the specific heat capacity is used, the amount of heat absorbed or released is cut in half.

Answers: 1
Another question on Physics

Physics, 22.06.2019 00:40
Electroplating is a way to coat a complex metal object with a very thin (and hence inexpensive) layer of a precious metal, such as silver or gold. in essence the metal object is made the cathode of an electrolytic cell in which the precious metal cations are dissolved in aqueous solution. suppose a current of 480.ma is passed through an electroplating cell with an aqueous solution of agno3 in the cathode compartment for 46.0 seconds. calculate the mass of pure silver deposited on a metal object made into the cathode of the cell. round your answer to 3 significant digits. also, be sure your answer contains a unit symbol. ×10μ
Answers: 3

Physics, 22.06.2019 15:30
What are the similarities & differences between a thermistor and a light dependent resistor in physics?
Answers: 2

Physics, 22.06.2019 19:30
A47.2 g block of copper whose temperature is 480 k is placed in an insulating box with a 91.8 g block of lead whose temperature is 200 k. (a) what is the equilibrium temperature of the two-block system? (b) what is the change in the internal energy of the two-block system between the initial state and the equilibrium state? (c) what is the change in the entropy of the two-block system? the heat capacities of copper and lead are 386 j/kg·k and 128 j/kg·k, respectively.
Answers: 1

Physics, 22.06.2019 23:30
Which of the following forces require physical contact? tension air resistance friction magnetic force applied force
Answers: 3
You know the right answer?
Describe how the amount of heat released or absorbed related to specific heat capacity and amount of...
Questions

Computers and Technology, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01



History, 12.10.2020 01:01




Mathematics, 12.10.2020 01:01




Mathematics, 12.10.2020 01:01

Social Studies, 12.10.2020 01:01

Biology, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

English, 12.10.2020 01:01


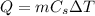
 is the variation of temperature.
is the variation of temperature.