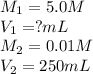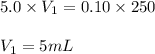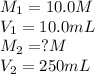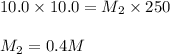Chemistry, 26.06.2019 19:00 tyneshiajones124
(a. what volume of 2.50 m lead(ii)nitrate solution contains 0.0500 mol of pb2+? ( b. how many milliliters of 5.0 m k2cr2o7 solution must be diluted to prepare 250 ml of 0.10 m solution? ( c. if 10.0 ml of a 10.0 m stock solution of naoh is diluted to 250 ml, what is the concentration of the resulting stock solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
(a. what volume of 2.50 m lead(ii)nitrate solution contains 0.0500 mol of pb2+? ( b. how many milli...
Questions

Biology, 19.03.2021 02:10



Mathematics, 19.03.2021 02:10


Mathematics, 19.03.2021 02:10



Computers and Technology, 19.03.2021 02:10





Biology, 19.03.2021 02:10


History, 19.03.2021 02:10

History, 19.03.2021 02:10

Mathematics, 19.03.2021 02:10

Mathematics, 19.03.2021 02:10

Mathematics, 19.03.2021 02:10

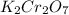 solution is 5 mL
solution is 5 mL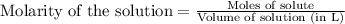
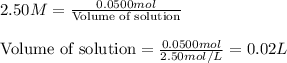
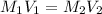 ......(1)
......(1)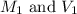 are the molarity and volume of the concentrated solution
are the molarity and volume of the concentrated solution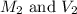 are the molarity and volume of diluted solution
are the molarity and volume of diluted solution