
Chemistry, 01.08.2019 01:20 jademckinziemea
For the second-order reaction below, the initial concentration of reactant a is 0.24 m. if the rate constant for the reaction is 1.5 x10–2 m–1s–1, what is the concentration of a after 265 seconds? 2a --> b + crate = k[a]20.12 m0.19 m0.95 m4.0 m5.2 m

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2


Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 08:20
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
You know the right answer?
For the second-order reaction below, the initial concentration of reactant a is 0.24 m. if the rate...
Questions


History, 24.09.2019 14:10





English, 24.09.2019 14:10


English, 24.09.2019 14:10

History, 24.09.2019 14:10


History, 24.09.2019 14:10



Biology, 24.09.2019 14:10





![\frac{1}{[A]}=\frac{1}{[A]_{0}}+kt](/tpl/images/0156/3924/a6d73.png)
![[A]_{0}](/tpl/images/0156/3924/48818.png) is intital concentration of A and k is rate constant.
is intital concentration of A and k is rate constant.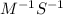 and t is 265 S
and t is 265 S![\frac{1}{[A]}=\frac{1}{0.24}+(0.015\times 265)](/tpl/images/0156/3924/91240.png)



