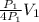Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
The pressure of a gas constant temperature is increased by a factor od 4 by what factor does the vol...
Questions



Mathematics, 21.04.2021 23:50



Business, 21.04.2021 23:50



Mathematics, 21.04.2021 23:50

Mathematics, 21.04.2021 23:50

History, 21.04.2021 23:50

Mathematics, 21.04.2021 23:50



Chemistry, 21.04.2021 23:50



Mathematics, 21.04.2021 23:50