
Chemistry, 22.09.2019 03:20 shealynh52
Considering the limiting reactant, what is the mass of iron produced from 80.0 g of iron(ii)oxide (71.55 g/mol) and 20.0 g of magnesium metal? feof)+ mg() fe)mgo6) a) 62

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
Considering the limiting reactant, what is the mass of iron produced from 80.0 g of iron(ii)oxide (7...
Questions

English, 15.10.2019 01:00


English, 15.10.2019 01:00








History, 15.10.2019 01:00



Mathematics, 15.10.2019 01:00


Spanish, 15.10.2019 01:00




Mathematics, 15.10.2019 01:00

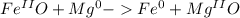
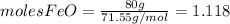 and
and 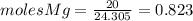 the molecular weight of Mg (24.305) can be readed in the periodic table of elements.so we divide the moles by stoichiometry number (number in front of each compound in the equation) in this case is 1 for both reactants (that is we need 1 mol of FeO and 1 mol of Mg to produce 1 mol of Fe).The lower number obtained was 0.823 for Mg, so Mg is the limiting reactant.
the molecular weight of Mg (24.305) can be readed in the periodic table of elements.so we divide the moles by stoichiometry number (number in front of each compound in the equation) in this case is 1 for both reactants (that is we need 1 mol of FeO and 1 mol of Mg to produce 1 mol of Fe).The lower number obtained was 0.823 for Mg, so Mg is the limiting reactant.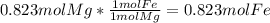 ). To convert from mol of Fe to grams of Fe we would multiply by the molecular weight of Fe
). To convert from mol of Fe to grams of Fe we would multiply by the molecular weight of Fe 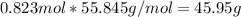 (molecular weight of Fe is readed in the periodic table of elements). So it is produced 45.95 g of iron
(molecular weight of Fe is readed in the periodic table of elements). So it is produced 45.95 g of iron

