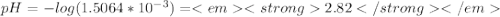Chemistry, 07.10.2019 17:30 justachelseafan
Weak acids and bases are those that do not completely dissociate in water. the dissociation of the acid or base is an equilibrium process and has a corresponding equilibrium constant. ka is the equilibrium constant for the dissociation of a weak acid and kb is the equilibrium constant for the dissociation of a weak base. what is the ph of a solution that has 0.125 m ch3cooh and 0.125 m h3bo3? ka of ch3cooh = 1.8 × 10−5 and ka of h3bo3 = 5.4 × 10−10 answer unselected 4.74 unselected 5.64 unselected 2.82 unselected 2.52 unselected

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Weak acids and bases are those that do not completely dissociate in water. the dissociation of the a...
Questions

Computers and Technology, 05.02.2021 01:20

Mathematics, 05.02.2021 01:20

History, 05.02.2021 01:20




Mathematics, 05.02.2021 01:20

Mathematics, 05.02.2021 01:20

English, 05.02.2021 01:20

Mathematics, 05.02.2021 01:20


Mathematics, 05.02.2021 01:20

Mathematics, 05.02.2021 01:20


Mathematics, 05.02.2021 01:20

Mathematics, 05.02.2021 01:20



Mathematics, 05.02.2021 01:20

Social Studies, 05.02.2021 01:20

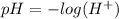 Equation (1)
Equation (1)
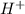 , because the proton is transferred to
, because the proton is transferred to 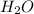 to form hydronium ions,
to form hydronium ions, 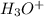 . Thus, we can determine the pH of the solution, finding the molar concentration of
. Thus, we can determine the pH of the solution, finding the molar concentration of 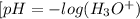 Equation (2)
Equation (2)
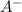
![k= \frac{[H_3O^{+}][A^{-}]}{[HA][H_2O]}](/tpl/images/0297/5192/1a08e.png) Equation (3)
Equation (3)
![k_a=K[H_2O]= \frac{[H_3O^{+}][A^{-}]}{[HA]}](/tpl/images/0297/5192/38c8c.png) Equation (4)
Equation (4)
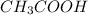 :
:
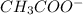

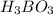 :
:
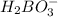
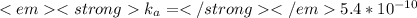
![1.8*10^{-5} =\frac{[x][x]}{[0.125-x]}](/tpl/images/0297/5192/6d8ce.png) Equation (5)
Equation (5)
![5.4*10^{-10} =\frac{[y][y]}{[0.125-y]}](/tpl/images/0297/5192/98489.png) Equation (6)
Equation (6)![x = [H_{3}O^{+}] = 1.49*10^{-3}](/tpl/images/0297/5192/56324.png)
![y = [H_{3}O^{+}] = 1.64*10^{-6}](/tpl/images/0297/5192/edda0.png)
![[H_{3}O^{+}]](/tpl/images/0297/5192/71b58.png) is (x+y)
is (x+y) 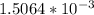 . Replacing this concentration in equation 2, we get:
. Replacing this concentration in equation 2, we get: