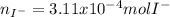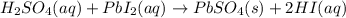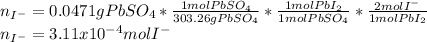Chemistry, 23.03.2020 21:30 Dericktopsom
When H2SO4 is added to PbI2, a precipitate of PbSO4 forms. The PbSO4 is then filtered from the solution, dried, and weighed. If the recovered PbSO4 is found to have a mass of 0.0471 g, how many moles of iodide ions were in the original solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3



Chemistry, 23.06.2019 07:00
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
You know the right answer?
When H2SO4 is added to PbI2, a precipitate of PbSO4 forms. The PbSO4 is then filtered from the solut...
Questions

Mathematics, 15.12.2019 00:31

Physics, 15.12.2019 00:31

Business, 15.12.2019 00:31

History, 15.12.2019 00:31


History, 15.12.2019 00:31

History, 15.12.2019 00:31

English, 15.12.2019 00:31


Biology, 15.12.2019 00:31

Mathematics, 15.12.2019 00:31




Biology, 15.12.2019 00:31




Mathematics, 15.12.2019 00:31