
Chemistry, 24.03.2020 17:41 adriantheclashroyaln
A 32.5 g iron rod, initially at 22.4 ∘C, is submerged into an unknown mass of water at 63.0 ∘C, in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 59.7 ∘C. Part A What is the mass of the water? Express your answer to two significant figures and include the appropriate units.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1
You know the right answer?
A 32.5 g iron rod, initially at 22.4 ∘C, is submerged into an unknown mass of water at 63.0 ∘C, in a...
Questions

History, 01.07.2020 15:01

Social Studies, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01

Biology, 01.07.2020 15:01




Mathematics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01

English, 01.07.2020 15:01

SAT, 01.07.2020 15:01






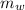 = 39.18 gm
= 39.18 gm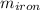 = 32.5 gm
= 32.5 gm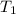 = 22.4°c = 295.4 K
= 22.4°c = 295.4 K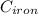 = 0.448
= 0.448 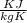
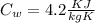
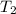 = 336 K
= 336 K 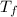 = 59.7°c = 332.7 K
= 59.7°c = 332.7 K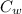 (
(

