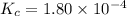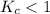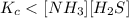Chemistry, 26.03.2020 21:33 demienarravo
The equilibrium constant, Kc, for the following reaction is 1.80×10-4 at 298 K. NH4HS(s) NH3(g) + H2S(g) This reaction is Reactant favored at equilibrium. Enter PRODUCT or REACTANT. The concentrations of NH3 and H2S will be at equilibrium. Enter HIGH or LOW.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 1.80×10-4 at 298 K. NH4HS(s) NH3(g) + H2...
Questions



Biology, 16.03.2020 22:43

Mathematics, 16.03.2020 22:43


Mathematics, 16.03.2020 22:43


Social Studies, 16.03.2020 22:43


Mathematics, 16.03.2020 22:43


Health, 16.03.2020 22:43

Mathematics, 16.03.2020 22:43




Chemistry, 16.03.2020 22:43


Mathematics, 16.03.2020 22:43

Mathematics, 16.03.2020 22:43

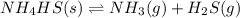
![K_c=[NH_3][H_2S]](/tpl/images/0565/8253/ff5ed.png)
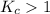 ; the reaction is product favored.
; the reaction is product favored.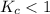 ; the reaction is reactant favored.
; the reaction is reactant favored.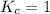 ; the reaction is in equilibrium.
; the reaction is in equilibrium.