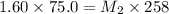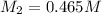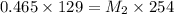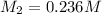Chemistry, 07.04.2020 23:01 iamcuriousdelip08rmf
A 75.0 mL aliquot of a 1.60 M solution is diluted to a total volume of 258 mL. A 129 mL portion of that solution is diluted by adding 125 mL of water. What is the final concentration? Assume the volumes are additive.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
A 75.0 mL aliquot of a 1.60 M solution is diluted to a total volume of 258 mL. A 129 mL portion of t...
Questions


Mathematics, 12.03.2020 05:28




Mathematics, 12.03.2020 05:28

History, 12.03.2020 05:28


Computers and Technology, 12.03.2020 05:28






Mathematics, 12.03.2020 05:28


Mathematics, 12.03.2020 05:29



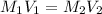
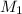 = Molarity of stock solution = 1.60 M
= Molarity of stock solution = 1.60 M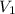 = volume of stock solution = 75.0 ml
= volume of stock solution = 75.0 ml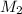 = molaity of diluted solution = ?
= molaity of diluted solution = ?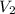 = volume of diluted solution = 258 ml
= volume of diluted solution = 258 ml