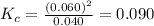Chemistry, 05.05.2020 18:04 AgentPangolin
At high temperatures, carbon reacts with O2 to produce CO as follows: C(s) O2(g) 2CO(g). When 0.350 mol of O2 and excess carbon were placed in a 5.00-L container and heated, the equilibrium concentration of CO was found to be 0.060 M. What is the equilibrium constant, Kc, for this reaction

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
At high temperatures, carbon reacts with O2 to produce CO as follows: C(s) O2(g) 2CO(g). When 0.350...
Questions





Mathematics, 16.04.2021 01:00

Social Studies, 16.04.2021 01:00




Mathematics, 16.04.2021 01:00

Mathematics, 16.04.2021 01:00

Chemistry, 16.04.2021 01:00

Mathematics, 16.04.2021 01:00

Business, 16.04.2021 01:00

Mathematics, 16.04.2021 01:00

Mathematics, 16.04.2021 01:00



Computers and Technology, 16.04.2021 01:00

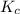 is 0.090.
is 0.090.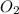 =
= 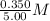 = 0.0700 M
= 0.0700 M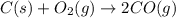
![K_{c}=\frac{[CO]^{2}}{[O_{2}]}](/tpl/images/0641/0273/4df57.png) , where [CO] and
, where [CO] and ![[O_{2}]](/tpl/images/0641/0273/9a638.png) represents equilibrium concentration of CO and
represents equilibrium concentration of CO and ![[CO]=2x=0.060](/tpl/images/0641/0273/522d6.png)