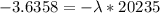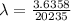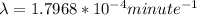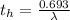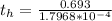Be sure to answer all parts. A freshly isolated sample of 90Y was found to have an activity of 2.2 × 105 disintegrations per minute at 1:00 p. m. on December 3, 2006. At 2:15 p. m. on December 17, 2006, its activity was measured again and found to be 5.8 × 103 disintegrations per minute. Calculate the half-life of 90Y. Enter your answer in scientific notation.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 12:30
What would most likely be the transmittance of a 0.70 m solution of solute a?
Answers: 1

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3
You know the right answer?
Be sure to answer all parts. A freshly isolated sample of 90Y was found to have an activity of 2.2 ×...
Questions


Mathematics, 22.03.2020 05:00



Mathematics, 22.03.2020 05:00

History, 22.03.2020 05:01

Mathematics, 22.03.2020 05:01


Mathematics, 22.03.2020 05:03




English, 22.03.2020 05:04



Mathematics, 22.03.2020 05:04

Mathematics, 22.03.2020 05:04

Mathematics, 22.03.2020 05:04



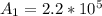 per minute
per minute 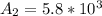 per minute
per minute 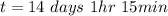
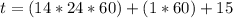
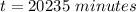
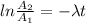
 is the rate constant
is the rate constant ![ln [\frac{5.8 * 10^{3}}{2.2 *10^{5}} ] = - \lambda * 20235](/tpl/images/0634/6329/6ece6.png)