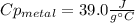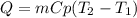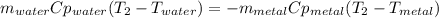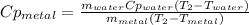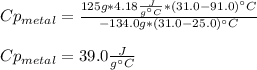Chemistry, 22.09.2020 04:01 jakiyahporter0817
A 134.0 g sample of an unknown metal is heated to 91.0⁰C and then placed in 125 g of water at 25.0⁰C. The final temperature of the water is measured at 31.0⁰C. Calculate the specific heat capacity of the unknown metal. Specific Heat of water is 4.18 J/g*C pls answer as quickly as possible

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
A 134.0 g sample of an unknown metal is heated to 91.0⁰C and then placed in 125 g of water at 25.0⁰C...
Questions


Advanced Placement (AP), 06.03.2020 19:23




History, 06.03.2020 19:24

Mathematics, 06.03.2020 19:24



Mathematics, 06.03.2020 19:24

Mathematics, 06.03.2020 19:24

Computers and Technology, 06.03.2020 19:24








Mathematics, 06.03.2020 19:25