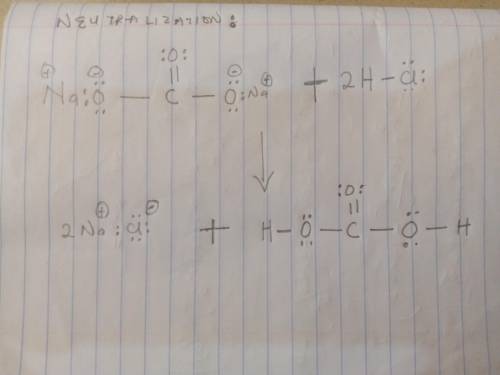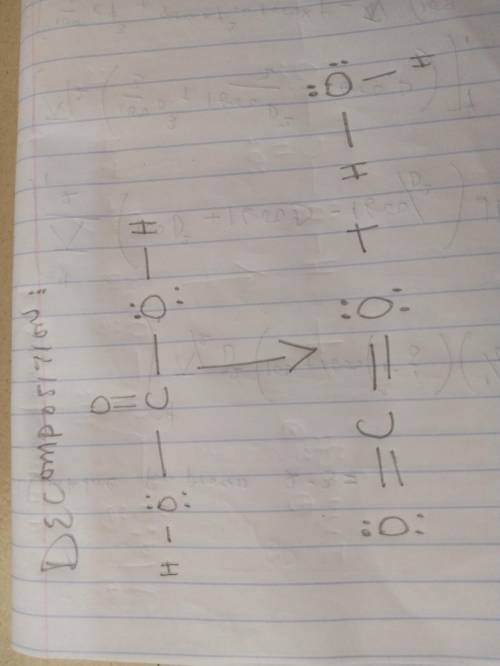Sodium bicarbonate is used to neutralize the remaining HCl at the end of the reaction. The initial products of this reaction are carbonic acid and sodium chloride. Carbonic acid then decomposes into carbon dioxide and water. Write a balanced chemical equation for each of these processes using Lewis structures (no molecular formulas).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1


Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3
You know the right answer?
Sodium bicarbonate is used to neutralize the remaining HCl at the end of the reaction. The initial p...
Questions


Mathematics, 01.01.2021 21:40

Mathematics, 01.01.2021 21:40



Computers and Technology, 01.01.2021 21:40

Mathematics, 01.01.2021 21:40


Mathematics, 01.01.2021 21:40

English, 01.01.2021 21:40



Physics, 01.01.2021 21:40

Biology, 01.01.2021 21:40





Mathematics, 01.01.2021 21:40