
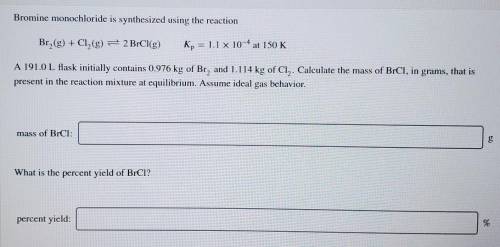
Bromine monochloride is synthesized using the reaction Br2(g) + Cl2(g) = 2 BrCl(g) Kp = 1.1 x 10^-4 at 150 K A 191.0 L flask initially contains 0.976 kg of Br2 and 1.114 kg of Cl. Calculate the mass of BrCl2 in grams, that is present in the reaction mixture at equilibrium. Assume ideal gas behavior.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3
You know the right answer?
Bromine monochloride is synthesized using the reaction Br2(g) + Cl2(g) = 2 BrCl(g) Kp = 1.1 x 10^-4...
Questions







Computers and Technology, 17.07.2020 01:01




English, 17.07.2020 01:01



Mathematics, 17.07.2020 01:01








