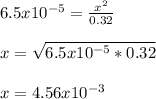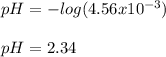Chemistry, 29.03.2021 22:00 underswap25
Benzoic acid, HC6H5CO2,is a monoprotic acid(only one H+ ionizes)with a Ka=6.5×10^-5. Calculate [H+] and the pH of a .32M solution of benzoic acid. PLEASE ANSWER.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
Benzoic acid, HC6H5CO2,is a monoprotic acid(only one H+ ionizes)with a Ka=6.5×10^-5. Calculate [H+]...
Questions



Mathematics, 09.07.2021 22:00













Health, 09.07.2021 22:00




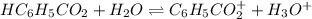
![[H_3O^+]=[H^+]](/tpl/images/1228/3574/877c7.png) , we can set up the equilibrium expression in terms of
, we can set up the equilibrium expression in terms of  (reaction extent) to obtain:
(reaction extent) to obtain:![Ka=\frac{[C_6H_5CO_2^-][H_3O^+]}{[HC_6H_5CO_2]} \\\\6.5x10^{-5}=\frac{x^2}{0.32-x}](/tpl/images/1228/3574/4456d.png)