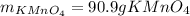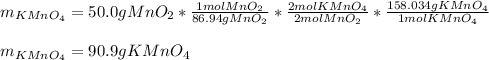Chemistry, 07.06.2021 05:50 lordcaos066
A company manufacturing KMnO4 wants to obtain the highest yield possible. Two of their research scientists are working on a technique to increase the yield.
Both scientists started with 50.0 g of manganese oxide (MnO2).
What is the theoretical yield of potassium permanganate when starting with this 50.0 g MnO2?
The equation for the production of potassium permanganate is as follows:
2 MnO2 + 2 KOH + O2 → 2 KMnO4 + H2
You must show all work to receive full credit.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
A company manufacturing KMnO4 wants to obtain the highest yield possible. Two of their research scie...
Questions

Biology, 12.09.2019 04:30

History, 12.09.2019 04:30



Social Studies, 12.09.2019 04:30

Biology, 12.09.2019 04:30

Mathematics, 12.09.2019 04:30

Mathematics, 12.09.2019 04:30


Mathematics, 12.09.2019 04:30

Mathematics, 12.09.2019 04:30







Mathematics, 12.09.2019 04:30


English, 12.09.2019 04:30