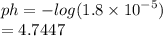b.9.3

Chemistry, 08.06.2021 07:30 seannalove6168
What is the pH of a solution with [H+] concentration of 1.86 x 10-5?
a.5.37 x 10-10
b.9.3
c.4.7
d.1.86 x 10-5

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
What is the pH of a solution with [H+] concentration of 1.86 x 10-5?
a.5.37 x 10-10
b.9.3
b.9.3
Questions

English, 25.03.2021 23:50



Geography, 25.03.2021 23:50





Mathematics, 25.03.2021 23:50


Mathematics, 25.03.2021 23:50

Mathematics, 25.03.2021 23:50






Mathematics, 25.03.2021 23:50


Biology, 25.03.2021 23:50

![pH = - log [ {H}^{+} ]](/tpl/images/1366/5000/6c1cf.png)