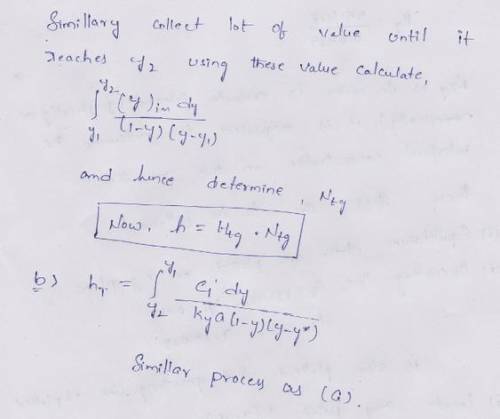Engineering, 28.03.2020 03:20 jenkinjack7654
A gas stream contains 4.0 mol % NH3 and its ammonia content is reduced to 0.5 mol % in a packed absorption tower at 293 K and 101300 Pa. The inlet pure water flow is 68.0 kgmol/h and total inlet gas flow is 57.8 kgmol/h. The tower diameter is 0.747 m. The film mass-transfer coefficients are k’ya=0.0739 kgmol/s. m3 .molfrac and k’xa= 0.169 kgmol/s. m3 .molfrac. Using the design methods for dilute gas mixtures,
(a) Calculate the tower height using k’ya.
(b) Calculate the tower height using K’ya.

Answers: 2
Another question on Engineering

Engineering, 03.07.2019 15:10
Heat is added to a piston-cylinder device filled with 2 kg of air to raise its temperature 400 c from an initial temperature of t1 27 cand pressure of pi 1 mpa. the process is isobaric process. find a)-the final pressure p2 b)-the heat transfer to the air.
Answers: 1

Engineering, 04.07.2019 18:10
Water at 70°f and streams enter the mixing chamber at the same mass flow rate, determine the temperature and the quality of the exiting stream. 0 psia is heated in a chamber by mixing it with saturated water vapor at 20 psia. if both streams enters the mixing chamber at the same mass flow rate, determine the temperature and the quality of the existing system.
Answers: 2

Engineering, 04.07.2019 19:10
In general, how do thermosetting plastics compare to thermoplastics in mechanical and physical properties?
Answers: 3

Engineering, 04.07.2019 19:10
Acircular aluminum shaft mounted in a journal is shown. the symmetric clearance gap between the shaft and journal is filled with sae 10w-30 oil at t 30°c. the shaft is caused to turn by the attached mass and cord. develop and solve a differential equation for the angular speed of the shaft as a function of time.
Answers: 2
You know the right answer?
A gas stream contains 4.0 mol % NH3 and its ammonia content is reduced to 0.5 mol % in a packed abso...
Questions




English, 26.09.2019 14:00


Spanish, 26.09.2019 14:00



Mathematics, 26.09.2019 14:00


History, 26.09.2019 14:00

History, 26.09.2019 14:00


Biology, 26.09.2019 14:00

History, 26.09.2019 14:00

Chemistry, 26.09.2019 14:00



Social Studies, 26.09.2019 14:00