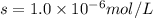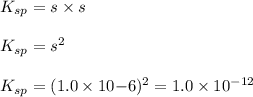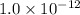agbr = 7.3 x 10-7

The molar solubilities of the following compounds (in mol/l) are:
agbr = 7.3 x 10-7
agcn = 7.7 x 10-9
agscn = 1.0 x 10-6
when these compounds are arranged in order of decreasing ksp values, what is the correct order?
agcn > agscn > agbr
agbr > agcn > agscn
agscn > agbr > agcn
agcn > agbr > agscn

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2


You know the right answer?
The molar solubilities of the following compounds (in mol/l) are:
agbr = 7.3 x 10-7
agbr = 7.3 x 10-7
Questions


Mathematics, 14.04.2020 19:27

Physics, 14.04.2020 19:27






Mathematics, 14.04.2020 19:27

Mathematics, 14.04.2020 19:27



Computers and Technology, 14.04.2020 19:27







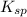 is
is 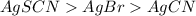
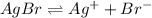
![K_{sp}=[Ag^{+}][Br^-]](/tpl/images/0399/7545/d2a23.png)
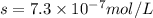
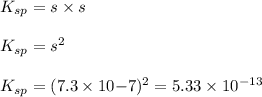
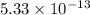
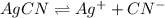
![K_{sp}=[Ag^{+}][CN^-]](/tpl/images/0399/7545/d7da5.png)
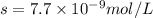
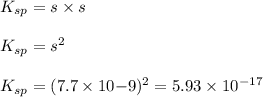
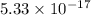
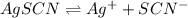
![K_{sp}=[Ag^{+}][SCN^-]](/tpl/images/0399/7545/55108.png)