Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3


Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
Consider the reaction for the combustion of benzene: 2 C6H6(g) 15 O2(g) ---> 12 CO2(g) 6 H2O(l) W...
Questions


Mathematics, 20.10.2020 09:01


Business, 20.10.2020 09:01

Health, 20.10.2020 09:01


Mathematics, 20.10.2020 09:01


English, 20.10.2020 09:01



Arts, 20.10.2020 09:01


Mathematics, 20.10.2020 09:01



Mathematics, 20.10.2020 09:01



Mathematics, 20.10.2020 09:01

 .....(1)
.....(1)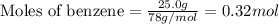
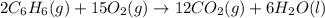
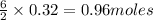 of water
of water